|
March 2022
0ur dear friends,collaborators, colleagues and supporters,
Still in tears and deep pain and sadness. Thank you for helping me to survive hour by hour day by day without my NENAD. Thank you for your support during these most difficult period of our lives.
Although still ill, I am ready to help all colleagues working with Nenad MarkPap® and continue his legacy that cervical cancer can be prevented and no one woman should die from it.
With warmest regards,
your Nenadica
September 2021
Dear colleagues and friends, this is Olivera-Nenadica sending greetings to you from the hospital.
I've shared with you our Markovic foundation and library. I've already started to prepare reagenes, so that you can see how easy it is to do the test.
We have spent years to make it easy, so that a low-trained person can do it, and women in remote, rural parts of the world can be protected with real0 screening of asymptomatic women (not when they start to have symptoms and treatment is more difficult).
Dr. Nenad Markovic's dream to help millions of women, globally, has become a reality.
Now, my dear friends, allow me to address Nenad in our native language.
Moj voleni najmili Nenade. Evo hocemo da ovekovecimo tvoj rad. To ti zasluzujes. Ziveli smo skromno, radili za opste dobro. Eto da se sada ovekoveciI Markpap Nenadov test.
Njaimilij moj ljubavi moja jedina, ljubavi zivota moga, sreco moja, ponos moj, u zivotu nisam videla i srela velicanstvenog coveka kao ti ode nam prerano, oh koliko sam te zeljna koliko mi nedostajes. Oh, Boze, nema zivota bez tebe.
Tvoja Nenadica
July 2021
Message from Dr. Olivera Markovic
Dear friends, family, collaborators and followers,
This is Olivera who is sending you a photo that we prepared for the anticipated Drs. Nenad and Olivera Markovic Foundation and Markovic Library.
|

|
We planned to collect materials that are located in four rooms full of scientific papers, published and draft results, reviews, lectures, patents and books. I planned even a small laboratory with ready-made reagents to do manual MarkPap® tests and enjoy simplicity where method was brought for even low-trained technicians to perform.
|
|
I wanted to donate microscopes, fax machines, refrigerators, even one of my pianos for relaxation. But it was not meant to happen now. Tragedy took place and we do not have with us brilliant professor Markovic any longer.
Let us thank him for his great contribution in science, healthcare, education, social health benefits.
MarkPap® technology has advanced now in MarkPap® System™ with Home-Digital MarkPap®. Self-sampling, which is a part of MarkPap® system, enables women in rural parts of the world to have screening even if they cannot visit a doctor, or have cultural sensitivity to having a pelvic exam, or other reasons (see citations). The percentage of women screened with MarkPap® Home-Digital™ is much greater, and self-sampling at home is particularly important during pandemics.
|
MarkPap® Digital™ also enables transmission of files from point of care (POC) to hospitals for final diagnosis. Only MarkPap® biomarker positive slides should be sent for Pap digital diagnosis. Biomarker negative slides are returned and women go home for the next year testing (see gallery). Complicated positive slides or samples could be submitted for further diagnosis in the United States.
|

|
MarkPap® system is finally helping the everlasting problem of cervical cancer screening - real screening of asymptomatic women - to be solved, and technology to be introduced not only in China, India and Europe, but to the whole world. This is a mission of the Global Academy for Women's Health, too.
This is Dr. Markovic's legacy that we should continue. Please write to me at info@bioscicon.com. I am still in the hospital greeting you from my hospital bed and a wheelchair.
Dr. Nenad was wonderful father to his children, providing them with the highest level of education and raising them in family values, honesty, fairness and independence in making their decisions with their own mind and principals, not affected by bad people around them.
Professor Markovic was also admired by his patients. When we used to go to congresses in former Yugoslavia, delegations of patients with their families would come to greet him from all parts of the country for what he has done for them.
Let us continue his legacy. The great Dr. Nenad would say, "where I stop, you continue; what I could not do you will".
For me, there are no words to say, I would give my life to see him just for a few seconds again. How can I thank him for a million days of perfect happiness? Most sincere love and devotion, like a beautiful, shiny crystal without any flows. My dearest, you were the most wonderful man I have ever met and seen in my whole life. Brilliant intelligence, huge professional and general knowledge (history, religion, life), he was like a walking encyclopedia. Firm, decisive, guided by principals, not by fear, always calm, polite and honest. Honesty was the hallmark of his personality.
Oh my dearest Nenad, I cannot describe the depth of my pain and sadness of this tragedy. Mili moj, voljeni Nenade, ljubavi zivota moga, sreco moja, radosti moja.
In order to express admiration to my Nenad, I decided to adapt an old custom from South Yugoslavia - to name a spouse by the first name of her husbamd, too. In my case it would be Olivera Markovic-Nenadica. I decided to proudly carry this name to the end of my life. We will remain forever two people with one shadow.
|
June 2021
Message on Passing of Professor Dr. Nenad Markovic
With deep pain and sadness we announce the passing of professor Dr. Nenad Markovic, president and CEO of BioScicon Inc, Rockville, MD.
Dr. Markovic was a brilliant scientist who authored over 300 scientific publications, articles and books, presented invited lectures, reviewed numerous scientific articles and book chapters. His own most recent book - "What every woman should know about cervical cancer", 3rd Edition, was recently published by Springer.
Dr. Markovic invented and developed a new cancer testing platform, MarkPap®. MarkPap® is a biomarker-based, fast, accurate and affordable cervical cancer screening technology. This means PAP testing can be available to much larger population, and the everlasting problem of cervical cancer can be finally resolved.
He was also an FDA medical reviewer.
Dr. Markovich was a respected professor for over 30 years, teaching undergraduate, graduate and post-graduate studies. He was a full professor at 5 universities in US and Europe, teaching internal medicine, oncology, hematology, pathophysiology. He developed an English-speaking school (university) in Novy Sad (former Yugoslavia). He served in a number of executive administrative roles such as the president of the Yugoslavian cancer society, with international paerticipation.
Always nice and calm, ready to help, Nenad was a wonderful father and husband. He was my inspiration, the love of my life, my happiness. We had the most beautiful, happy life together for over 60 years. Two bodies and one shadow. I love you so much, my dearest Nenad. Zbogom naimilij moi.
February 2021
COVID-19: OPINION LEADERS, WHERE ARE YOU?
COVID-19 is a new disease. We all agree with it. But, this disease is almost a year among us. I cannot accept the lack of knowledge about it, at least lack of initiatives to describe the syndrome(s) of the disease and at least to evaluate the most popular symptoms of it and to help less knowledgeable people to protect themselves with better understanding what they are doing, and what they can expect.
Before this pandemic, I remember, there were famous professors writers of medical books, who always tried to understand the disease, to describe it correctly and to propose solutions, if not to cure the disease, but to improve the most disturbing symptoms, or to ameliorate the disease syndromes. Those people were known as opinion leaders. Where are they in case of COVID-19 pandemic?
I am a medical doctor, professor of several clinical subjects, and I do not see anything similar on the horizon of medical scientific literature. Media efforts are not sufficient. We need professionally written, individually responsible, comprehensive summaries of the disease and the recommendations on how to diagnose, treat and cope with it.
The most recent NIH initiative to support research (OTA-21-0158: Post-Acute Sequels of SARS-CoV-2 Infection Initiative: SARS-CoV-2 Recovery Cohort Studies) in the same area, is the first light in the tunnel of our illiteracy on COVID-19 disease and its long term natural history. But, using the NIH grants to fund research in unchartered fields is a long way to go. We and the public need something much faster.
We need the courageous opinion leaders to publish their current observations, to honestly say what they do not know, and to suggest the next steps toward the common goal: putting the COVID-19 pandemic under control and eventual eradication of the pathogenic virus.
We need these authors to write multiple monographs devoted to different aspects of the disease like: Definition, Epidemiology, Diagnosis (anamnesis, physical exam, laboratory assays, imaging diagnosis), Clinical staging (diagnosis, spread of disease), Therapy (protocols, the best choice) Recovery and Complications, Palliation, and Coping with the disease outcomes. These descriptions should be in the format that can be included into a text book written with a comprehensive approach and intended to serve as educational guidance for practitioners and users. Personal experience is also much appreciated.
Such articles by opinion leaders deserve to be published in the leading medical journals and magazines, as well as to be discussed at various scientific meetings. From our side, BioSciCon will open a page: “Opinions” for publishing individual contributions of value for better understanding and better management of this still new and insufficiently known disease COVID-19.
For more information, please write to the BioSciCon’s Customer Service at info@bioscicon.com, or call at 1 (240) 614-7128 M-F at Eastern working hours.
January 2021
Biomedical Science Consulting was incorporated in 1998 to serve as a formal business structure where the owners can legally convey their knowledge and experience to a larger audience than the number of patients they could treat individually working as medical doctors with individual patients. This life-changing decision was made when they realized of having an invention that can fight cervical cancer changing its natural path of development.
Soon, they learned about the un-obviousness and uniqueness of their invention, but also of the size of the social impact it may do globally if developed into products and services to be applied on huge global health care market permanently demanding for improvement of tools used for delivery of health services.
|
 |
The current pandemic COVID-19 has increased requirements for home-based testing, remote diagnosis, digital communications and early intervention based on accurate data. All these requirements are met in the palette of our new products: Home, Self-Sampling, Remote Diagnosis, Mobile MarkPap® test, all these included in the MarkPap System™, which is now in development. Click here to learn more.
BioSciCon, Inc. is the acronym of the full name of the company, Biomedical Science Consulting, Inc, and it is used for commercial purposes. The role is focused on the development of MarkPap Platform Technology products and services intended to help health care providers to reverse ever rising the mortality of cervical cancer curves and to provide tools for health care providers to sustain the negative mortality trends obtained with the new strategy, products and services.
This goal has been accomplished by developing and strengthening by patents a huge intellectual property and with market developing by speeding the awareness of the problem, and the strategies to achieve improvements (books and marketing material),
Today, BioSciCon, Inc. is a product specification developer for MarkPap Platform Technology's products and services. Currently, it has a new patent Application with 12 dependent claims in development. The estimated stock value of the whole program is calculated on hundreds of millions, inasmuch as the market value of its application could be in billions.
On the global awareness side, BioSciCon published two books, "What every woman should know about cervical cancer", Edition in 2008, 2nd Edition, 2017, 3rd Edition in manuscript and much scientific publications.The last is an abstract for the next EB FASEB Meeting 2021, entitled "Pandemics vs. Cancer smouldering epidemics".
All information above is intended to be announced during 2021 Cervical Cancer Awareness Month.
More details about MarkPap products are available and ready for sharing. But, to be part of it the interested parties must oblige the American business legal system and comply with technicalities as LOI (Letter of Intent), NDA (Non-Disclosure Agreement), MOU (Memorandum of understanding) MTA (material, transfer agreement) and many others.
You may obtain further information by contacting BioSciCon Customer Service Center
- By e-mail: info@bioscicon.com, or
- By phone: 1-240-614-7128
When leaving a voice mail, please start with a personal introduction.
Thank you for your interest and welcome to the BioSciCon website, www.bioscison.com
|
August 2020
BioSciCon, Inc. has been awarded
2020 Best of Rockville Award in Biotechnology Research and
Rockville Business Hall of Fame
BioSciCon, Inc. has been selected eleven years in a row for the 2020 Best of Rockville Awards for Biotechnology Research and now qualifies for the Rockville Business Hall of Fame.
In August 2020 because of the CORONA virus pandemic, the healthcare situation, including Maryland, drastically changed. The new priority has been defind, both in healthcare and Govermental demands.
|
 |
In order to meet those new requirements, BioSciCon has changed and upgraded its original MarkPap® Home-based Speimen Collection Kit to the level to include early detection of COVID-19 and to keep cervical cancer preventive screening in an advanced mode: MarkPap® HB SS-K, a Home-based Self-sample Collection Kit for early detection of COVID-19 (contmination, disease), HPV (infection, disease), detection of early epithelilal lesions (cervical, oral, anal) which if not treted or removed could develop into cancer.
On August 16, 2020 it was applied to USPTO, PPA 63066247.
Partners are invited for further development.
Rockville Honor Program Honors the Achievenent. Click this link to read more.
|
January 13, 2020
BioSciCon, Inc. is sponsoring the development of MarkPap platform technology, strategy, programs and services. In the recent period, BioSciCon has accomplished a big part of Intellectual Property development. The comprehensive device MarkPap System™ which includes 12 modules has already been applied for US Patent. At the end of last year, on November 7, 2019, the first of the individual modules has been applied for utility patent.
The begging of the series of finalizing intellectual property achievements is starting the new process of seeking funds for monetization and commercialization for all these new products and services.
On behalf of this trend BioSciCon is using this opportunity to announce a search for partner(s) who would be willing to help financially and intellectually on this road toward successful social and financial ending. Social impact will be measured by the reduction of mortality of women from cervical cancer, and the financial with the Profitability Index which is calculated to be above 1.2.
For more details, please read the prior announcements on this site.
November 2019
BioSciCon's Intellectual Property is growing
In November 2019, the Application, elaborating One Claim, the Complex, meaningful biomarker MEDYKO has been filed. It is an acronym, composed of ME (metabolic change), DY (dysplasia, diskariosis), KO (koilocytes, HPV Disease, pure prognosis).
Patent Application procedure has started.
Please consult our book, What every woman should know about cervical cancer, Springer 2017 (59).
August 2019
BioSciCon's Intellectual Property is growing
A new patent Application MarkPap System™ has been in procedure since 2018. It contains 1 compound medical device comprising 12 modules covering new products and services derived from the Parent Patent CAP-PAP Test. Mobile Home Pap Test is among the latest products and services.
The new complex biomarker MEDYKO evolved further from the originally patented CAP-PAP Test.
The new Self-sampling Kit will dramatically increase the outreach among women in rural areas of the world and in the countries where cultural, tradition barriers prohibit women to visit gynecologists.
The IT/ Mobile technologies are being further developed, specialist is not necessary to be present at POC. That makes the system infrastructure independent and can be performed everywhere, in the car, on the ship, in rural parts of India, China, Africa.
The MarkPap System™ remained a biomarker-based, telemedicine enabled, low-cost, simple, affordable, accessible, equitable, infrastructure independent platform technology.
When implemented this technology will be among those which can bring right care at the right place on the right time for lower cost. This is currently the only promise for mass cervical cancer screening worldwide.
The MarkPap Home Mobile System is a hope that solving the everlasting problem of Global Cervical Cancer Screening worldwide is not far anymore.
May 2019
It is the National Women's Health Week.
Please read GAWH Policy Statement for Cervical Cancer Screening 2019/20
Cervical Cancer Strikes Back
Cervical cancer is known from ancient times as the important killer of women from malignant diseases. For thousands of years this disease is resistant to any therapy except to an early removal of suspect lesions, and... show more
Introduction
Cervical cancer is known from ancient times as the important killer of women from malignant diseases. For thousands of years this disease is resistant to any therapy except to an early removal of suspect lesions, and in recent two Centuries, following the increase in the world population and better communication enabling interactions between different people, this fatal disease has gained recognition of having natural grown of mortality rate for about 10% per year.
In the middle of 20th Century, the mortality of cervical cancer worldwide and in the US was about 30 per hundred thousands. In countries with any type of screening this number was reduced to one third, and in the US, the introduction and nationwide application of Pap test screening has helped American healthcare providers to further reduce the mortality rate to about 3 per a hundred thousand women, reduction of more than 85%. It was expected that with all new improvements (LBP, HPV) mortality will be reduced to less than 1 per a hundred thousand women.
Unfortunately, it did not happen. Now, in 2019, the mortality of cervical cancer in the US is back to the best results obtained with the classic Pap test only, or 3 per thousand. CDC has recently reported that new incidence of cervical cancer in the US is predicted to be 13,200 for 2019, or about 4000 higher than in the best period before. Obviously cervical cancer is present and active. The ACS has published their newest guidelines recovering the Pap test and cytological screening to protect the accuracy of prevention. Hologic and Quest have followed this pattern and are reviving the cytological screening. Even Roche, the manufacturer of COBAS is mentioning the value of microscopic analyses and cyto-diagnosis.
What happened? Something went wrong. But what? What to do to prevent errors to repeat, and to keep the trend for reduction of mortality as predicted.
We went back to the history of the Pap test and, we think, we were able to identify some periods when application of Pap test was exposed to changes that affected its successful progression as the “best cancer screening test available.”
1. Early period – 1945 – 1996
In 20th Century, American gynecologists used vaginal fluids to collect exfoliated squamous cells to make vaginal smears and to search for cervical cancer. It was a successful approach reducing the mortality, but was not accurate method.
In 1945 Dr. George Papanicolaou introduced his test using cleaning cervix and then excoriating cervical superficial cells. It was much better technique providing cleaner smears and better vision of cellular structures enabling the investigators to detect suspicious cells that could develop into cervical cancer. The basic cytological classification: no changes, mild changes, intermediate and severe changes ending with cervical cancer, was established and served the purpose for years. In this period, by sending women with positive test to colposcopy and further diagnosis including removal of suspicious lesions, American health care providers reduced further the cervical cancer mortality rate to 3 per thousand, the rate that was sufficient to remove the cervical cancer from the list of 10 most frequent cause of death ofAmCervicalerican women.
2. The Bethesda NIH Consensus Conference on Cervical Cancer: TBS –surrogate classification. Twenty percent false negatives. Call for improvement.
However, in this “golden “ period for Pap test, several cytopathology laboratories lost legal suites for medical malpractice because they missed to identify false negative Pap smears of women who died of cervical cancer although being diagnosed as negative Pap.
In the meantime, scientific information accrued that the standard Pap test, in a form as applied, might have about 20% false negative readings due to problems with specimen collection options, specimen crowded with infectious cells, and difficulty for clear reading of DNA changes.
The National Consensus Conference was called to resolve those problems with consensus if not with pure evidence.
The Conference ended with a success.
Pap smear test was confirmed as “the best cancer screening test available”
The 20% false negative rate was allocated to the specimen collection
The call was open for new technology to improve this insufficiency.
Finally, the Bethesda System classification was developed to change the standard cytological classification with a modern version using more strange words: nil (no disease), BCC (benign cervical cell changes including reactive);ASCUS ) abnormal squamous cells with undetermined significance; LSIL (Low grade squamous intraepithelial lesion); HSIL (High grade cervical intraepithelial lesion) and ICC (invasive cervical carcinoma).
Although this new classification looks much better I(more precise] than the old one, it
really is only a surrogate list of endpoints to rank the success/failure of the new methods for cervical cancer screening. The robust, outcome based (life/death) method was put aside. This was the fist change affecting measuring the accuracy of the mortality rate caused by cervical cancer.
3. Liquid based specimen collection
Soon ThinPrep Pap test was introduced by Cytyc Corp., and SurePath Pap test by TriPath Imaging Inc. Both are based on the specimens collected in their special cell preservative solution and their apparatus (processor) for automatic slide preparation before staining. Papanicolaou stains remained unchanged.
Both companies brought slides very much appealing to cytopathologists –they were free of detritus, reduced number of small inflammatory cells, squamous cell much better distributed over the slide, transparency preserved and intracellular components clearly visible. It is easy to diagnose TBS changes.
Although both companies advertised that their solution contains much more cells from the sample, what is true, in practice, not frequently more than one slide is processed. Using another sample will rocket the price per investigation. The cost for Pap test exam has already doubled with trend to rise.
The solution was found in reduction of sampling frequency (once in three years). This was another, but very serious damage to the reduction of the false negative rates. Obviously, it was a nonrealistic solution.
4. Rising cost – extension of mandatory periods for screening
Annual screening was recommended to reduce 20% false negative rates.Within three years this rate could be reduced to 5% or the acceptable statistical error rate. Extending the periods between screenings is affecting false negative rate negatively – increasing the probability of false results to up to 50% which is unacceptable for any test.
5. HPV enthusiasm for eradication of the virus etiology
In the beginning of 21st Century the HPV has arrived. Indeed, it was approved as test to detect HPV virus particles which could cause HPV disease, could make the persistent long term disease and could finally cause cervical cancer to appear. This FDA born terminology was correct. That any persistent inflammation can produce changes on DNA structure which may turn into cancer has been a knowledge known for hundreds of years. But, the public wishes, the long starving for good news cancer healthcare providers and the public, cut the central part of this paragraph, and used for advertisement only the fist phrase ands the last conclusion – the test that could detect cervical cancer.
This how testing HPV and HPV vaccines became the Holy Grail trophy for most of us.
In order to meet this market requirements, the test providers, Becton Dickinson, Quest, Hologic, Qiagen, LabCoprs and the other made another change that indeed discounted the role of the classic Pap test. They proclaim that their tests are better than the Pap test for detecting CIN 2/3 changes. This is not the screening for which the Pap test was developed, this is a diagnosis of an early cervical cancer. It means that the current methods have abandoned the cervical cancer screening principle of the Pap test and compared screening with diagnostic. This is the final strike against the “best cancer screening available.” It went to history.
Suddenly, the Pap test became irrelevant, the screening of cervical cancer eliminated, detection of early signs and the lesion that could develop into cervical cancer were left outside the major examination. The lesions were left to grow and were not eliminated. The environment for punishment was setup.
And the punishment came.
As predicted, the cervical cancer is not giving up so easy. In few years, cervical cancer is coming back and kills women again. Was it necessary to happen? Or, what to do now?
In 2019, CDC projected 13,200 new cases of cervical cancer in US, about4000 more than a couple of years ago. Since the mortality rate is staying the result is in increased number of deaths every year. Is it what we planned and hoped for? No, the reality is just opposite of the hopes.
Based on this information, our conclusion has been that the original Pap test had its problems, but the new technologies introduced as improvements have not done what was expected from them, and the cervical cancer is coming back.
6. Current evidence-based sobering
Now, we have to accept the evidence that the cervical cancer and mortality rate are coming back, due to our inadvertent errors. Cancer is not under our control yet, and it follows it own rules. We have to adapt our strategy, tools and services and to stop its growth, not being exulted by the false hopes. The treatment must follow the natural history, navigate it, but cannot change it without a major, breakthrough, events – which did not happen.
However, the cost of the current Pap test has grown enormously and must be put under control. And kept in range of below $15.00 - $30.00 per test. Our estimate is that this price can be achieved with quantity discount and the new, improved Pap test can be applied globally using the principle, "rich pay for poor."
7. A light in the tunnel? Maybe!
MarkPap Platform Technology (products and services) in 2019, because available for licensing. It is a new IP comprising of a composite independent utility patent application with 12 revise and derived modules as optional dependent claims indicating to the opportunity to be developed into a set of new individual patents.
To meet the major requirement discussing above the new application shows ability:
To screen for cervical cancer with wider gamut of biomarker (metabolic for premalignant alterations, morphological for DNA confirmation of malignant progress, and HPC disease to shape up the prognosis, if not intervened on time.
To increase the screening outreach above 51% of all women at risk. The Self-sampling kit enabling women to collect specimens at home and to send it to laboratory for diagnosis is a the guarantee of a large if not huge outreach among women at risk to submit their specimens for examination; Diagnostic kit with MEDYKO option and automatic staining offers a standard procedure and accurate results obtained fast because of short turnaround, and HPV disease confirms the malignant outcome.
To improve the accuracy, reduce false negative rates and minimize the coast using IT principles. With ITTHC network making the entire cycle(collecting specimen, obtaining results, completing the management of women after the results, can be done fast (within hours) accurate, interaction among experts, low cost (IT technology involved).
Epilog
The old definition of cancer is still valid: Cancer is an unstoppable growth of abnormal cells, destroying the normal tissue on its path or distribution within the body. This natural history may be affected by many agents, the growth slowed, diverted, affected cell type changed, but the fatal outcome cannot be avoided if the tumor is not entirely eliminated from the body.
This is where we stand now, and what we have to do.
show less
November 2018
During the 9th Annual Clinical Diagnostics and Research Conference, on November 15, 2018, Dr. Olivera Markovic delivered a lecture entitled:
CERVICAL CANCER, EVERLASTING WORLD HEALTH CHALLENGE
The on-line conference was organized by Labroots, a promoter of on-line education and sponsor of Continuing Medical Education (MCE) (www.labroots.com).
To attend the lecture, free registration is required here.
Upon registration and logging on to the site, click this link and press Launch button.
August 2018
Best for Women's Health Promotion 2018 - Maryland
Following on from your recent nomination acceptance in the Global Health and Pharma Healthcare and Pharmaceutical Awards 2018, and our dedicated research stage, the judging panel have made their final decisions and I am delighted to inform you of the outcome.
BioSciCon, Inc has been awarded:
Best for Women's Health Promotion 2018 - Maryland
"It is our desire to reward those who demonstrate dedication to furthering the advancements within healthcare and pharmaceutical services and products. For this reason, we have developed a merit-based judging process providing entrants with the opportunity to share their proudest moments, and greatest achievements."
(From the Award Announcement)
July 2018
Dr. Markovic and BioSciCon, Inc were nominated for the Healthcare and Pharmaceutical Award 2018, by Global Health and Pharma.
Global Health and Pharma is a global information sharing platform & a multi-disciplinary members community, established to enhance communication networks & collaboration across all themes and disciplines within main categories: Human, Animal & Environmental Health, Academia, Industry, Public Bodies & Health Systems, Governments & Policy Makers, Funding Agencies & Groups.
June 2018
Drs. Olivera and Nenad Markovic contributed to the EB 2018 Meeting in San Diego, CA with the report "Is HPV Vaccination Secure Protection of Women With or Without Cytologic Cancer Screening." FASEB J. 32, 1, Supplement, April 2018. Read more...
May 2018
Prof. Dr. Nenad Markovic, President of BioSciCon, Inc. and MarkPap Pacific, LLC presented the recent development of BioSciCon's MarkPap® Technology System on Maryland - Anhui (China) Promotion Week in Rockville, MD
"Maryland - Anhui Promotion Week is the outcome of a meeting of the Governor Larry Hogan and Anhui Party Secretary Li Jinbin last summer. During that meeting the Governor and Party Secretary agreed that the 38-year old Sister State relationship between Maryland and Anhui, established in 1980, is deserving of a focus, celebration and re-commitment to the deep and significant economic trade, education, and cultural relationship between Maryland and Anhui Province" - citation from the Announcement.
The celebration featured displays and information of Maryland businesses, among them BioSciCon,Inc., and MarkPap Pacific LLC, which was incorporated to commercialize BioSciCon's MarkPap® Platform Technology in PR China and Greater Pacific area.
April 2018
National Cancer Control Month
April is National Cancer Control Month.
According to CDC, "Cancer control is a journey. It starts with healthy choices like a good diet, vaccines, proper sun protection, and cancer screening—all things that can help make it less likely you will get some kinds of cancer. If you do get cancer, the path shifts to helping you find the best treatment, and then finding support through the rest of your life as a cancer survivor."
Global Academy for Women's Health and BioSciCon, Inc. with their dedicated medical doctors, scientist and educators are working tirelessly and passionately to contribute to the cervical cancer control in the world. This grave, deadly disease is completely preventable and curable IF detected on time by screening. Unfortunately more than 300,000 women die per year from this preventable disease in the 21st Century. Most of them live in the developing countries, where screening is available to only less than 20% of women at risk. There are countries in Africa that the percentage of women who have preventive screening is less than 5% (Uganda). So, almost all women getting cervical cancer (cc) are destined to die, instead, after small early intervention, to continue full productive life. These are somebody's mothers spouses sisters, daughters who die unnecessarily, leaving the whole family devastated.
Why is this happening? In spite of money invested by governments, industry - multi-billion corporations and NGO's, and in spite of the awareness about the danger from cervical cancer, the situation has not been significantly improved until now. There is still 10% increase in mortality. Even in the US, the perfect example of successful prevention with Pap test, most recently cc started to increase among minority populations. WHO is searching for new strategies and new models to fight cc.
Based on analysis of the situation in 57 counties in the world, more than 2/3 of the world population, understanding the real barriers of cc prevention (screening), we describe the new strategy how to fight the challenge of increasing trend of cc. We develop a new strategy and the new tools to enable this strategy. The platform technology includes new biomarkers (Medyko), intensive use of IT technology, particularly image information exchange of files and protocols for global networking, to achieve fast, accurate and low-cost diagnosis via mobile and Internet connection between scattered POCs and medical centers and with a protocol to enable turn-around of data within hours and timely intervention-removal of suspicious lesions that can develop into cc. The MarkPap technology, which is biomarker-based and IT (wired and mobile enabled) is fast, accurate and affordable, infrastructure-independent, accessible, and culture sensitive platform. As such it is aimed for mass cervical cancer screening worldwide.
Since outreach for screening is very important part of the strategy, a Self-Sampling Kit is made available for women to take a sample at home and send it to the POC. This enable woman living in rural areas, far from medical institutions of any kind, to participate in screening, and for those who, because of tradition/religious issues, are not allowed to visit gynecologist. The program is aimed to start in parallel developing local infrastructure enabling sustainability of the new developments. This is exactly according to the recommendations of WHO and Dr.Tedros Adhanom Ghebreyesus, Director General who is acquainted with our work and supports our initiative.
The MarkPap® platform technology is known in India. It has been tested at AIIMS, All Indian Institute for Medical Sciences in New Delhi. Joint papers are published in Indian prestigious journals and the result presented on International meetings. Also, it has been applied at four major medical schools in India where Prof Markovic mentored faculties for their doctorate theses. Indian scientists cite the MarkPap technology as "the future of cervical cancer screening." Currently, there are more than 70,000 deaths from cervical cancer in India. Only less than 20% women get screening. 280 million women are left behind. Collaboration continues.
The MarkPap® patented platform technology (1995) is now widely practiced in China, but under "different names": NewMarkPap, NM Pap, FSC-811. Our Chinese "colleagues" are describing this technology as "innovative technology for the benefit of mankind".
We are now starting to launch the technology In Africa. Cervical cancer is number is the main cause of death from malignant diseases in women.
Our experience is summarized in the Second updated and extended edition of the book "What very woman should know about cervical cancer", published by Springer in 2017. The book is distributed worldwide by Springer and its associates.
This is a monograph-type text book with more than 500 pages, plenty of illustrations, explanation and guidances for professional and non-professional audiences. For professionals, the book provides analysis of current needs and demands (Ch.5), a proposal for a new strategy on how to fight cc (Ch.6), and the new tools to enable this strategy Ch.7): http://www.springer.com/biomed/cancer/book/978-1-4020-6936-9.
Our book is also aimed for public audience, for women to be their companion when they are sick and afraid where to go and seek help, and when they are healthy how to stay healthy (health diet, stress release strategies, etc).
The book is also available on www.amazon.com in electronic version and individual chapters can be downloaded.
It seems that the momentum is coming now. Governments in some developing countries already started to include cervical cancer screening in the yearly women's check up. The WHO is searching for new approaches suggesting new technologies to be made sustainable in those countries. Our book is just on time.
Today, there are 2.4 billion women at risk from cervical cancer who need help. Huge space for tremendous social impact and financial benefit. Making money doing good is the motto in our Consortium of social entrepreneurial companies and a non-profit organization.
Everybody is welcome.
We are trying to develop alliances, strategic partnerships to continue our efforts. Donations are appreciated. Investors are welcome (equity investment, licensing, royalty).
Drs. Olivera & Nenad Markovic legacy continues.
March 2018
Updated BioSciCon, Inc. Flyer
 |
Please click the image
to view the flyer. |
February 2018
During the month of February, when BioSciCon and Global Academy for Women's Health celebrate the National Cancer Prevention Month, we are notified about the acceptance of our presentation at the Experimental Biology 2018 FASEB Meeting entitled "Is HPV Vaccination Secure Protection of Women Without or With Cytological Cancer Screening".
October 2017
For the second time, BioSciCon, Inc. and Dr. Olivera Markovic were announced winners for the Corporate Vision Magazine Business Woman Awards (2017)
Discussing the awards, Rachel Devonport, Awards Coordinator, commented: "Women represent around half of the global workforce and are a key contributor to the corporate world; despite this, these contributions are often overlooked. As such it is my privilege to showcase the very best women in business across the corporate landscape, and I would like to offer them my congratulations as well as wish them the best of luck as they look to the future."
July 2017
Awards and Recognitions
Dr. Olivera Markovic was nominated to receive Business Woman of the Year Award 2017
Hosted by Corporate Vision Magazine. "Across the globe, within a vast number of sectors, women continue to go above and beyond to establish themselves as being a significant part of the business world. From healthcare to pharmaceuticals... these awards are purely based upon merit.."
Dr. Olivera Markovic has been selected for 2017 Marquis Lifetime Achievement Award
This award came after a decade of multiple bibliographic citations of Dr. Markovic's work in several editions of Marquis Who is Who.
"We are pleased to announce that Marquis Who's Who has selected you for our official 2017 Albert Nelson Marquis Lifetime Achievement Award. You have been selected to receive this prestigious award as a result of your hard work and dedication to your profession. Congratulations!"
BioSciCon, Inc. has been selected for the 2017 Best of Rockville Award for Biotechnology Research by the Rockville Business Recognition Award Program
"Welcome to the Rockville Business Hall of Fame! BioSciCon, Inc. is among a very small group of companies that have won the Best of Rockville Award for eight consecutive years. This distinction has qualified BioSciCon, Inc. for the 2017 Rockville Business Hall of Fame."
April 2017
Drs. Olivera and Nenad Markovic contributed to the Experimental Biology National Meeting, EB Chicago, 2017 with a report on Cervical Cancer and HPV: Dilemmas and Resolution via Biomarkers. See Reference #60.
January 2017
| At the beginning of the Cervical Cancer Awareness Month, we are happy to announce that the second, updated and extended edition of our book "What every woman should know about cervical cancer" was published by Springer. More... |
 |
December 2016
2016 Awards and Recognitions for BioSciCon, Inc.
- NATIONWIDE (DOMESTIC)
We announce with pride that Dr. Olivera Markovic and BioSciCon, Inc. were awarded 2016 Corporate America Elite Award, New York:
Best Woman-Owned Biotechnology Company - Mid-Atlantic.
"The 2016 American Businesswoman Elite Awards are designed to place strong headed women in the spotlight and to give recognition to those most deserving. We have searched the country for strong headed women who continue to shatter glass ceilings on a daily basis and become a significant part of the business world, we feel those individuals deserve to be recognized, and awarded, for their continuous hard work and talents."
Jazmin Collins, Awards Co-ordinator, expressed her pride in the award winners: "Women play a significant part in the corporate market, and as such it is a great honor to be able to turn the spotlight on our amazing winners and showcase their talent and dedication. I would like to wish them the very best of fortunes going forward."
http://www.corporateamerica-news.com
- INTERNATIONAL
We are also honored to receive Businesswoman Awards 2016 by Corporate Vision Magazine, England
Dr. Olivera Markovic and BioSciCon, Inc. were awarded Best Woman-Owned Biomedical Science Consultants-Maryland.
"Corporate Vision is dedicated to working around the clock to shine a spotlight on the brightest, best performing and most deserving companies and individuals from around the business world.We’re fiercely passionate about recognizing outstanding achievement, game-changing innovation and stellar performance, and all of our awards are carefully tailored to provide detailed and in-depth analysis of the very best each market, industry, sector and region has to offer.We take choosing our winners very seriously and every single one is chosen on merit. As we said, we’re only interested in recognizing the very best so, no matter how big a business is, nobody can buy their way to success ..."
http://www.corp-vis.com
- LOCAL RECOGNITION
BioSciCon, Inc. has been selected for the 2016 Rockville Business Hall of Fame by Rockville Award Program.
"BioSciCon, Inc. is among a very small group of companies that have won the Best of Rockville Award for seven consecutive years in the Biotechnology Research category. This distinction has qualified BioSciCon, Inc. for the 2016 Rockville Business Hall of Fame. To commemorate your inclusion in this elite group an exclusive Hall of Fame Award, available only to Hall of Fame inductees, has been created...These exceptional companies help make Rockville area a great place..."
http://rockville.localibestof.com
November 2016
Labroots Virtual Meetings
Drs. Olivera and Nenad Markovic participated in two recent Labroots Virtual Meetings:
- Could we treat patients with cancer, not cancer with patients. Labroots Cancer Research and Oncology Virtual Meeting, October 5-6, 2016.
See the Abstract, one-page poster and the video "Kinetics of nanoparticles accumulation visualizing potential biomarkers" in Reference #57 (References on this web site)
- POC's past, present and future. IT empowering POC's. A need to equal health care for all. Labroots Clinical Diagnostic and Research Virtual Meeting, Nov 2-3, 2016.
See the Abstract and one-page poster in Reference #58.
September 2016
It is to announce that Dr. Olivera Markovic is elected Adjunct Professor of Biological Sciences at the University of Maryland Baltimore County. She is currently teaching classes in Graduate Master Degree Program in Biotechnology at UMBC, USG. This is the fifth professorship granted to Dr. Markovic, after Medical Faculty, University in Belgrade, former Yugoslavia, Medical College of Pennsylvania/Drexel University in Philadelphia, PA, Georgetown University, in Washington, DC and American University in Washington DC.
August 2016
A new composite marker for cervical dysplasia
MEDYKO is a new upgraded MarkPap® biomarker. It is an acronym of ME (metabolic biomarker--acid phosphatase), DY (pathocytological signs of dysplasia) and KO (koilocyte sign of HPV disease).
It combines signs of pre-cancerous condition, cancer disease and cancer prognosis; altogether metabolic, pathocytological and HPV diagnostic assay.
Read more...
MOTHER'S DAY 2016
Message from the President
Mothers of the World, Foundation of the Family and its Guiding Force, our Heroes -- Happy Mother’s Day!
However, sadly, today in the 21st Century, thousands of women still die from preventable diseases. One example is cervical cancer, whichis completely preventable and curable if detected on time, but it is stillone the major women’s killer in the world, particularly in the developing countries. What to do about it, how to help women aroundthe world?
How to prevent it? The answer is: With cervical cancer screening to detect early changes of cervical cells that may develop into cancer and to educate women how to take better care of their health. Unfortunately, after 70 years of Pap test availability, the ultimate goal – to reverse of cervical cancer trends globally – has been achieved partially – in developed countries, only. In most of developing countries, the outreach for preventive cytological screening is less than 10%. Screening for risk factors such as HPV has contributed, but still not sufficiently.
Is global mass cervical cancer screening possible today? Our answeris: YES, but with new tools. With the fast development of IT technology application in medicine (e-Health & M-Health) and diagnosis at distance (telemedicine), with new biomarkers improving the staining procedure of Pap test, this can happen and thousands of Points-of-Care (POC) may become screening units for affordable cost.
BioSciCon’s MarkPap® platform technology belongs to this new generation of Pap tests. A low-trained technician at the POC in the remote sites can process the specimen with customer-friendly MarkPap® Kit, locate suspicious cells highlighted by the biomarker and transmit microscopic images, via MarkPap® Networking System,to experts for instant diagnosis. Home MarkPap® Specimen collection kit will further increase accessibility and make the test culture-sensitive. See www.bioscicon.com.
The whole procedure should be completed while a woman is still on the premises, some smaller intervention can be performed at the POC, or a woman could be timely directed to a health care institution for further treatment (One-Day Pap test).
This biomarker-based, telemedicine-enabled, infrastructure independent, low-cost, simple, affordable, accessible, and culture- sensitive technology combined with wide education of women to increase awareness about prevention, may become a new tool for mass cervical cancer screening, a technology which can bring right care at the right place and at the right time for lower cost.
The MarkPap® technology is already widely implemented in China, with current efforts to be introduced in India. With targeting support, it could be introduced globally and save thousands of women’s lives.
May this Mother’s Day, 2016 be the beginning of the new era and the best greeting for all women in the world.
November 2015
BioSciCon Introduces the New Proprietary Biomarker
for Mobile Telecytopathology
BioSciCon, Inc. and the Global Academy for Women's Health have submitted an abstract for the 2016 Experimental Biology Annual Meeting in San Diego, CA , where intend to present their new proprietary biomarker for cancer diagnosis via global mobile telecytopathology.
BioSciCon, Inc. has been selected for 2015 American Leadership Award by the American Economic Institute, Washington, DC.
August 2015
BioSciCon, Inc. receives 2015 Best of Business Award for Rockville
Small Business Community Association recently selected BioSciCon, Inc. to receive 2015 Best of Business Award for Rockville, MD in the Small Business category.
July 2015
BioSciCon's MarkPap® [CAP-PAP™] Test in India
We have a long-standing collaboration with Indian scientists where the validity of the of the MarkPap test (CAP-PAP test) in detection of abnormal cells on cervical smears has been confirmed. Please see Publications 38, 51.
In the beginning of this year two more scientific papers were published on the subject in India.
1. Prof. Santwani with her team at the Department of Pathology, Shah Medical College, Jamnagar and Punjab Institute of Medical Sciences, published a paper "Study of CAP-PAP versus conventional Pap in suspicious cervical lesions" using MarkPap Kit with Combo Control Slides. The paper appeared in Int J Res Med 2015;4(1)102-108, concluding that the CAP PAP test could be the future of of cervical cancer screening. Click here to see the article
2. Prof. Nenad Markovic supported a doctoral candidate, Dr. Niranjan J, at the Videhu Institute of Medical Sciences in Bangalore, who performed a clinical trial, according to the design provided, confirming the value of CAP-PAP test and published the results in J Evidence Based Med&Healthcare, 2015; 2(6),714-23, 2015, "Cervical Acid Phosphatase; Evaluation as an adjuvant to papanicolaou smear screening in cervical cancer detection. Click here to see the article
India is a country with 70,000 deaths from cervical cancer per year, and below 10% outreach for the prevention from this disease. Out of 300,000M women at risk only 20M women are protected with preventive cancer screening. Cervical cancer is preventable and curable disease, IF detected on time.
But, new strategy is needed.
On June 11, 2015, Drs Olivera and Nenad Markovic met officially with Minister Counselor for Science and Technology Mr.Tarun Mohandra and Attache Mr. Kumar at the Indian Embassy in Washington, DC. The new Strategy for Fighting Cervical Cancer in India with MarkPap technology tools was presented and discussed. The Strategy has been forwarded to the Indian Ministry of Health.
Our book "What every woman should know about cervical cancer'( Springer, 2008) is popular in India. Recently, two Indian distributors joined the list: Delhi Book Store and Meltek Books.
June 2015
BioSciCon's MarkPap® Images in China
Original, copyrighted image of a specimen processed with MarkPap® Test, presented on BioSciCon's Gallery page, are plagiarized and published without permission at the website of the Chinese Anhui Science and Technology Co.
March 2015
MarkPap® in P.R. China
CAP PAP Test, USPTO #6,143,512 (issued in year 2000), MarkPap®, BioSciCon, Inc, USA is gaining popularity in China. It is sold under different names: NewMark-PAP, NM-PAP from Anhui Science and Technology Co., Ltd and FSC-811 from Anyon Biopharmaceuticals, Ltd, both from Hefei, Anhui Province, PR China.
Citation from www.ahnmst.com
(Google translate)
"Abnormal cervical squamous cell early screening kit" to detect chemical and enzyme modified Pap staining of cervical squamous cell early lesions improve the readability of abnormal cells, high sensitivity, shortened reading sheet time, low cost, which is very suitable for large-scale screening of cervical cancer, is suitable for use at all levels of medical institutions. "Innovative technology for the benefit of mankind."
November 2014
BioSciCon is now offering two MarkPap® Technology related new products for licensing/sale. Both are in patent pending phase.
1. BioSciCon Smartphone-microscopic Universal Adapter for Telemedicine
2. MarkPap® Self-Collecting vaginal/oral specimens Kit™ and Test for cancer screening
Please visit our Licensing Page for more information.
June 2014
BioSciCon, Inc. and its principals have been recognized, endorsed and selected as award recipient by business organizations, associations, media, bibliographical collections, etc. BioSciCon, acknowledges these awards and is honored by the recognitions.
Marquis Who's Who Publication Board recognized and certified Dr. Olivera Markovic for the 15-th jubilant year of being a subject of bibliographical record series of Marquis Who's Who. "Inclusion in which is limited to those individuals who have demonstrated outstanding achievements in their own fields of endeavor and who have, thereby, contributed significantly to the betterment of the contemporary society" (Plaque citation).
The 2013 Best of Rockville Award in the Biotechnology Research Category came to BioSciCon, Inc. from the US Commerce Association, given by the Rockville Award Program. According to the 2013 Rockville Award Program Press Release, "The Rockville Award Program was established to recognize the best of local businesses in the community. . . those companies that have shown the ability to use their best practices and programs to generate competitive advantage and long-term value."
In 2014 BioSciCon has qualified and is nominated for 2014 Rockville Business Hall of Fame.
May 2014
Please view an insert from the video, presented at the NIH Single Cell Analysis Investigators Conference in April 2014, Bethesda MD.
To read more, click here.
Single Cell Analysis: Measurement of Enzyme Kinetics in Single Cells
The video is an insert of the video presented at the NIH 2nd Single Cell Analysis Investigator Conference, Bethesda, MD, 2014. It presents a model for monitoring, recording and measurement on enzyme kinetics in single cells through the dynamics of the formation of the Final Reaction Product (FRP), an aggregate of nano molecules of colored, insoluble product accumulating gradually on the sites of enzyme activity. On the image analysis instrument screen, the growing product is seen as white shining signals, as presented on the figure below.
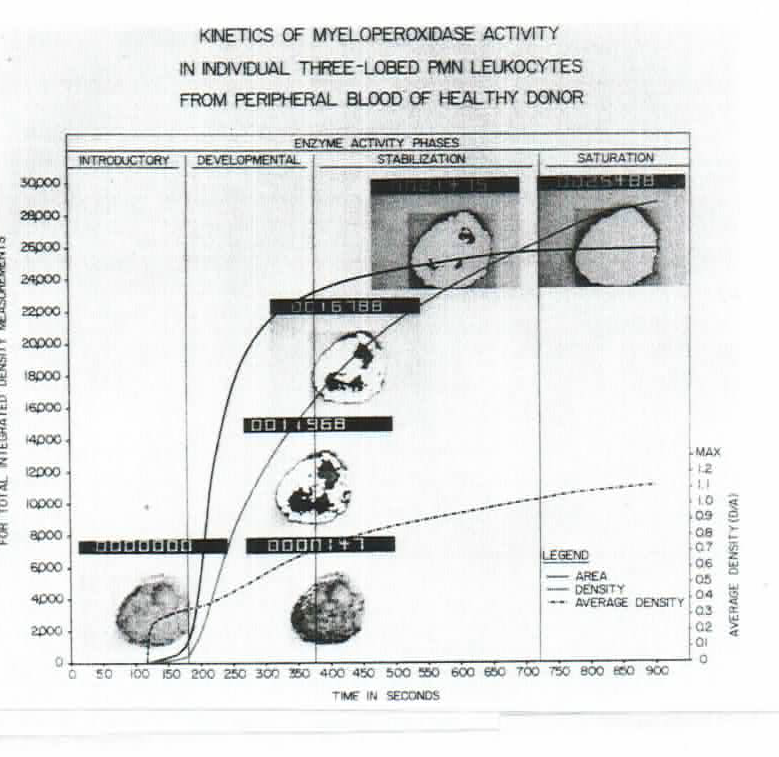 Measuring parameters are: Area, Density and Average Density of the FRP. Four phases of the reaction were described: Introductory, Developmental, Stabilization and Saturation phase. The Enzyme Unit is obtained from the density curve in the middle of the Stabilization Phase, estimated from the Area curve (O. Markovic.Assay of enzyme activity in single cells. Periodicum Biologorum 82,492, 1980).
Measuring parameters are: Area, Density and Average Density of the FRP. Four phases of the reaction were described: Introductory, Developmental, Stabilization and Saturation phase. The Enzyme Unit is obtained from the density curve in the middle of the Stabilization Phase, estimated from the Area curve (O. Markovic.Assay of enzyme activity in single cells. Periodicum Biologorum 82,492, 1980).
This course of the enzymatic reaction can be influenced by the introduction of modifiers (e.g., drugs) and the response can be recorded inside a single morphologically classified cells. More than 20 papers on the application of the new technology. To mention few, from the field of investigation of enzyme-targeted chemo-therapies: "Image processing assisted measurement of intracellular effect of enzyme-targeted drugs (Cell Vision, 2,71, 1995), A new assay of intracellular measurement of inosine monophosphate dehydrogenase activity: A guide for better selection of patients for enzyme-targeted chemotherapy (J Histochem Cytochem 42,23,1994). More references are available on request.
Although the work was well accepted and appreciated, it did not gain wide acceptance because it required expensive computerized image analysis system necessary to obtain quantitative information. However, since Single Cell analysis became recently very attractive field for new investigators, BioSciCon and the Global Academy for Women’s Health decided to work on the simplification of their original technology and make it more affordable for wide use.
The anticipated use of such device is by pharmaceutical industry for new drug development, in particular for new chemotherapeutic drug for regulating tumor tissue growth.
In prospective, such simplified devices could be used for personalized medicine and for prediction of the success of certain therapies for particular patients and diseases.
Hide details for May 2014.
March 2014
The participation of BioSciCon and the Global Academy for Women's Health on the January 2014 National Conference on Cancer Detection, Diagnosis and Treatment Technologies for Global Health, appeared in Hopkins News, March 2014.
Click here to read the article.
In February 2014 led by BioSciCon, Inc and Global Academy for Women's Health, and with participation of companies from LMIC counties, a project Proposal entitled Wireless Cervical Cancer Screening in Low and Medium Income Counties was submitted to the National Institutes of Health. Click here to read the Narrative of the Proposal.
February 2014
MarkPap India, LLC, a member of the BioSciCon Consortium, continued with implementation of the Strategy for Fighting Cervical Cancer in India, with simplified, affordable, telemedicine-empowered MarkPap® technology that can bring cervical cancer prevention in rural India, at the BoP, where it is most needed. Unfortunately, at present - in the 21st century - more than 70,000 women a year die in India from preventable cervical cancer.
Please visit our MarkPap India, LLC GUST web site.
In January 2014, led by BioSciCon, Inc and MarkPap India, LLC, sponsored by the Global Academy for Women's Health, project proposal entitled A Comprehensive Information Technology Healthcare Center for Cancer Screening in India was submitted to NIH. Twenty organizations from the US and India are committed to participate in this project. Please click here to read the Narrative of the Proposal.
January 2014
Global Academy for Women’s Health and BioSciCon participated in the 2014 NIH organized National Conference on Cancer Detection, Diagnosis and Treatment Technologies for Global Health in Bethesda, MD.
The Global Academy presented a review: ”Meeting WHO Recommendations for cervical cancer screening in developing countries.” The presentation was enriched with long experience the authors have gained working with Indian partners in India. Data presented are in support of the concept that the high tech medical devices could always be simplified to meet more limited requirements. One of the solutions to this task is using biomarkers for specific diseases and navigating all consequent procedures and tools through the scope of the selective biomarker.
According to WHO, Pap test remains the best cervical cancer preventive test. However, its classic version cannot be directly applied in the developing world primarily because of the cost and lack of infrastructure. Fortunately, the new biomarker-based Pap test, MarkPap technology products and services which are empowered with telemedicine for distant reading may provide a low-cost, simple, infrastructure independent, accessible, equitable and culture-sensitive opportunity to implement cytological cervical cancer screening all over the world, particularly in the developing world, where it is most needed (www.bioscicon.com).
In the subsequent discussion, Dr. Olivera Markovic emphasized, based on experiences in China and India, that the biggest barrier is not the cost, but lack of infrastructure at the Point-of-Care (e.g., qualified pathologist, trained technicians), which is most difficult to overcome and needs decades to be developed. Here, telemedicine, telecytopathology providing diagnosis on distance, is the method of choice. Particularly, mobile cell phone transmission of microscopic images from the Point-of-Care to the specialist. The whole procedure may take just few hours, and women could get diagnosis and initial treatment within one single day, while still on the premises.
The new ideas attracted a particular interest in the audience, which expressed interest for more information and for guidance on how to use these technological inventions in their environment.
BioSciCon’s had a technology video presentation, a slide show on ”Changing bioactivity of live cancer cell in vitro’” Drs. Markovic revived their single cell analysis technology for monitoring, recording and measuring the kinetics of enzyme activity in a single cell. The concept and model of this technology was developed many years ago, while they were working at NiH, using expensive microscopes and image analyzers. It is now simplified to make it available for researchers with lower resources. Having a possibility to measure the kinetics of intracellular enzyme activity provides a new chemo-sensitivity testing method, which may be used for screening many small molecules for their bioactive properties in search for new anticancer therapeutics.
Global Academy for Women Health and BioSciCon, together with collaborators from the US and India, submitted a grant proposal to NIH, entitled ” A comprehensive IT Telehealth Center for Cervical Cancer Screening in India”. This development, if supported, will open a prospective to screening, diagnosis and treatment of Indian women the same day they visit a Point-of-Care.
December 2013
Due to popular demand, BioSciCon has upgraded the Consulting Page. Please visit www.bioscicon.com/consulting.html.
August 2013
 |
The Global Academy for Women's Health, a non-profit affiliate of BioSciCon, Inc., acknowledges the contribution from Ms. Farkhondeh (Ferry) Sadeghi with her beautiful paintings. Ms Sadeghi is pharmacist, philanthropist and one of the most respected and active artists in the Washington Metropolitan Art Society. Her paintings are on display in the Offices of the Global Academy for Women's Health at the Johns Hopkins University, Montgomery County Campus, Rockville, MD. The photo displays her most recent gift to the Academy. |
July 2013
Pap Smear Test Can Be Made Available and Accessible to Women World-Wide.
It has been tested and repeatedly confirmed during BioSciCon's R&D of the proprietary platform technology under the brand name MarkPap®, which is biomarker-based and telemedicine-empowered. The products in the production pipeline are designed to be low-cost, simple, fast, accurate, accessible, equitable and infrastructure-independent, with selective intended application such as Mass Pap Smear Cervical Cancer Screening World-Wide.
Currently, BioSciCon is offering a set of three products from the MarkPap® pipeline for developing countries, particularly India: MarkPap® Test Reagent Kit (a set of reagents, controls and instructions for simple processing specimens by a low-trained technician at the Point-of-Care). MarkPap® Telemedicine Service (diagnosis at distance) and MarkPap® Self-Collection Home Kit (to allow women to take sample at home and send it to the lab for testing). Practically, it means that a women can take a sample at home and send it to the lab where a low-trained person can process the specimen with the simple Reagent Kit. The same person looks on the slide under the microscope searching for biomarker positive (red flagged) abnormal cells and transmits the images of those microscopic fields with a cell phone to specialists. The result could be obtained back within one hour. If implemented, this is how MarkPap® products could bring Pap test across the globe.
The products are ready for marketing, and sales are contingent to the local (in-country) regulatory requirements.
June 2013
We were present at the 18th International Congress of Cytology in Paris, where the paper entitled "Evaluation of Cytology Screening Strategies for Cervical Cancer In Resource Poor Setting" was presented. Please see the Reference # 51.
May 2013
BioSciCon, Inc. supported by the Global Academy for Women's Health is currently developing low-cost, simple devices for early detection of oral cancer. Oral cancer is common cancer and is among major health problems in the developing countries, particularly in India. To read more about this most recent effort, click on this link.
April 2013
BioSciCon, Inc. and its affiliates is continuing its effort for the development of low-cost, simple medical devices which are infrastructure independent and accessible in low-resource countries. Telemedicine (wired and wireless) provides powerful tools to achieve success. Please click here to read "IT Telehealth Center for Telecytopathology" that appeared in The FASEB Journal 2013, 27:874.20.
February 2013
The Global Academy for Women's Health has submitted Application to the US Indo Government funding for organizing the Symposium "Strategy for Reversing the Negative Trends of Increasing Prevalence and Mortality of Indian Women from Cervical Cancer." The Symposium is anticipated to be held in 2013 at the Johns Hopkins University, Montgomery County Campus in Rockville, MD. The Proposal for a comprehensive strategy to increase the outreach for preventive screening to 50% during the next 10-12 years is in preparation. It is expected that the negative trends of an increasing morbidity and mortality at the current outreach of 6% will start to decrease when reaching 50% point.
January 2013
In response to the WHO launched the Non-Communicable Disease Action Plan, BioSciCon's Consortium has concentrated on three topics, which are in our domain:
- Develop scientific strategies for cancer prevention and control.
- Develop standards and tools to guide the planning and implementation of interventions for prevention, early detection, treatment and care.
- Provide technical assistance for rapid, effective transfer of best practice intervention to developing countries.
The Global Academy provides assistance to the Consortium member, MarkPap India, LLC, in the Strategy for Fight Against Cervical Cancer in India (See Brochure-November 2012). BioSciCon has sponsored the development of tools to guide this Strategy.
November 2012
According to the World Health Organization, carcinoma of oral cavity is the sixth most frequent cancer among males in developing countries. Although it represents 2-4% of the malignancies in the western countries, it is estimated to be as high as up to 40% of male cancers in some areas of India.
Inspired by the Global Academy for Women' Health, the non-profit member of the BioSciCon's Consortium, BioSciCon, Inc., a social enterprise, continues its mission for development simple, low-cost medical devices suitable for the Point-of-Care with minimal infrastructure and and low-trained personnel in rural , low-resource areas in the world. MarkPap® proprietary platform technology is a typical example for a biomarker-based, telemedicine empowered, simple, affordable, accessible , infrastructure independent technology for early detection of abnormal cells in cervical specimens.
Recently, another medical device. from the same category, APE-100 was announced by BioSciCon for early detection of abnormal cells from oral cavity. Those patients who do not have access to medical institution can obtain oral specimen at home, using a simple Self-Collection Kit and mail it to the doctor’s office. APE-100 is in R&D phase of development.
October 2012
MarkPap India, LLC Brochure on the "Fighting Cervical Cancer in India"
Recently, MarkPap India, LLC, the whole seller of BioSciCon's proprietary MarkPap® products for India, has completed the package of products and services that can be used to reverse cervical cancer negative trends of increasing prevalence and mortality from cervical cancer in this country. MarkPap India has announced the news in its Brochure that can be retrieved by clicking on the link.
September 2012
| After feasibility testing, the concept for the Universal Adapter was cleared for patentability and a new patent application was filed with the USPTO. Please see images of the early phase of this development. |
|
|
June 2012
The Global Academy for Women's Health is strongly supporting the development of low-cost, simple medical diagnostic devices that could be used globally to alleviate the disparity in women's healthcare. Wireless health is providing a wide avenue to advance in this field, since 80% of the world's population lives in regions with mobile phone accessibility.
Read more about BioSciCon's efforts, supported by the Global Academy for Women's Health, to advance wireless telecytopathology (wireless transmission of microscopic images) from the Point-of-Care to specialists for instant diagnosis, that appeared in Hopkins News, June 2012.
May 2012
BioScicon and The Global Academy for Women's Health contributed to FASEB Experimental Biology Annual Meeting, 2012 with the following communications:
Is Mass Cytological Cancer Screening Possible Worldwide?
Universal Adapter for Telecytopathology
March 2012
The preparation of the Second updated and extended edition of “What Every Woman Should Know about Cervical Cancer" is announced in Hopkins News, February 2012.
December 2011
The renown international publisher Springer invited Dr. Nenad Markovic and Dr. Olivera Markovic to prepare the second, extended and updated edition of their book “What Every Woman Should know about Cervical Cancer." The first edition was published by Springer in 2008; it is well accepted and is distributed worldwide by Springer, Amazon, and affilited distributors.
August 2011
BioSciCon, Inc. participated at the 2011 NIH Annual SBIR/STTR Conference, held in June 2011 in Bethesda, MD, with the poster entitled: Capitalization of investing into medical device development on the health market.
BioSciCon Consortium participated at the NIH Conference on Cancer Detection and Diagnostics Technologies for Global Health . Bethesda, MD, August 2011 with the presentation entitled: Low cost medical devices combined with telemedicine reduce health disparities between developed and underdeveloped countries.
July 2011
ATTENTION
BioSciCon Consortium has decided to license some of its MarkPap® pipeline products for global cervical cancer screening. Interested parties, please send inquiries to info@bioscicon.com for more details.
April 2011
Dr. Olivera Markovic, Director of BioSciCon, Inc. provided an interview to the Virginia Telehealth Network on the current progress of telemedicine efforts of BioSciCon's Consortium to bring expert medical healthcare to the remote point-of-care locations in low-resource areas, where it is most needed.
Please visit the Virginia Telehealth Network web site.
January 2011
BioSciCon Consortium submitted Application for NIH US India Collaborative Program on Low-Cost Medical Devices.
December 2010
BioSciCon Consortium submitted Application to NIH for SBIR phase-1 Grant.
July 2010
BioSciCon, Inc. has been selected for the 2010 Best of Rockville Award in the Biological Research category by the US Commerce Association. Click here to read more.
May 2010
BioSciCon, Inc. is featured at Virginia Telehealth Network Web Site. BioSciCon, Inc is developing IT and Mobile Telehealth Center for Telecytopathology Services in Rockville, MD
January 2010
MarkPap Pacific, LLC is featured in Washington Post
MarkPap Pacific, LLC and its activities were featured in the Washington Post on December 28, 2010. Click here to read the Washington Post article.
November 2009
MarkPap Pacific, LLC signs a Trade Agreement with Chinese Partner
MarkPap Pacific LLC, a Company from the BioSciCon Consortium, signed a Trade Agreement with Chinese Partner to bring the MarkPap Test to China market: To sell minimum one million tests per year and to use telemedicine (telecytopathology) services during the next five years.
September 2009
BioSciCon and its companies on the forefront of mHealth current development
The mHealth denotes delivering health care services using mobile wireless communications that has recently emerged as a new viable option for providing health care in developing countries. The Year 2009 is proclaimed as the Year of mHealth. According to the United Nations Foundation, the mHealth is “a high reach, cost-efficient method for making healthcare more accessible, affordable and effective across the developing world.” Many regions in the world do not have access to the Internet, but 80% of the world’s population lives in regions with mobile phone accessibility. This is a huge market for the new mHealth methods, products and services, and a target for the Global Health Initiative and its followers.
BioSciCon’s Consortium of Companies is on the forefront of mHealth current development. We are developing a platform technology for wireless telecytopathology/telemicroscopy that uses mobile cellphone cameras for capturing microscopic images directly from the microscope in remote locations and wireless transmission of those images to distant centers for evaluation by professionals with or without interceptions of web-based digital-image networking systems. The system provides opportunity for the results to be returned to the end users within hours. We have a patentable discovery that could be a revolutionary solution for improving health service delivery and surveillance in the developing world where it is most needed.
The MarkPap® Wireless Telecytopathology™ is based on our prior experience with our proprietary MarkPap® Digital Telecytopathology™. Application of mobile phones for capturing and transmitting microscopic images directly from the microscope with cellphone camera is not an easy task, but we are aware of the challenges and the Wireless Telecytopathology Team is solving them. Please visit www.bioscicon.com/gallery.html to see the Gallery, the actual images obtained with a cellphone camera, applied for early detection of abnormal cells on cervical smears. The previous Gallery, the MarkPap Gallery, is created with a digital camera and it is possible to compare the quality of images between the two.
mHealth heralds a new era in medical communications and health service delivery and requires capable and well equipped multidisciplinary groups and teams devoting their energy to this task. Then the success is warranted.
August 2009
BioSciCon, Inc. becomes a Corporate Partner of Johns Hopkins University
BioSciCon, Inc. became officially a Corporate Partner of Johns Hopkins University, Montgomery County Campus in Rockville, MD. " We are extremely pleased to be able to meet the needs of this small R&D company on our campus, said Elaine Amir, Executive Director, Johns Hopkins Montgomery County "We've been long planning to create an incubator or accelerator on our campus, and the addition of BioSciCon to our campus is a first step in doing so". (Hopkins Happenings, July/August Issue, 2009).
March 2009
BioSciCon's MarkPap® Wireless™ Images for Telehealth
|
In August 2008, we introduced the MarkPap® Wireless™ technology. The technology is based on using standard microscopes and mobile cell camera phones for capturing and transmission of microscopic images to digital imaging systems in order to enable worldwide telemedical diagnosis of pathological specimens. Below is an example of MarkPap® images collected by (1) mobile cell phone camera and (2) digital camera with software for digital image analysis. It is obvious that these two images are comparable and that the image analysis or medical diagnosis could be performed interchangeably.
|
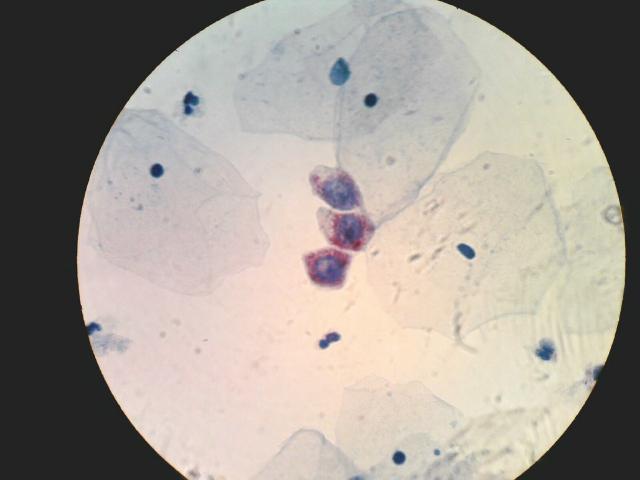 |
|
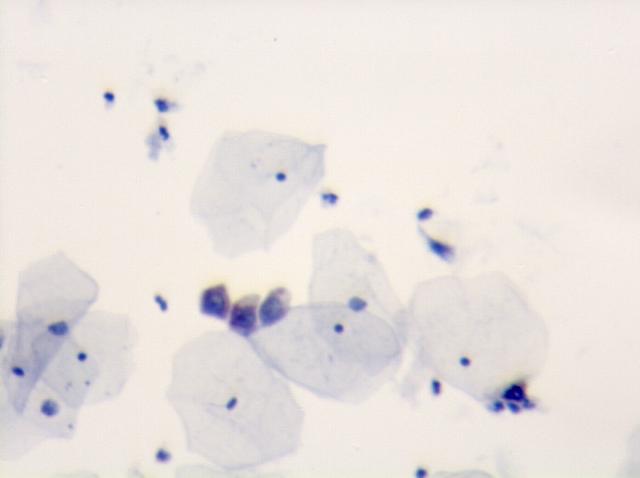 |
| Image 1 |
|
Image 2 |
|
Both images are taken from the same cervical specimen, processed with MarkPap® test, where abnormal cells were identified with the red biomarker. Normal cells are blue and do not contain the marker. The marker makes the important difference for identification of the medical condition preexisted in the specimen and visualized by the MarkPap® test. We believe that wireless telecytopathology will make a big advancement in global health (telehealth). Only a microscope and a cell phone will allow transmission of images for evaluation at distant places. BioSciCon is inviting scientists and licensing around the world to join their efforts and bring together this opportunity to the world for saving more human lives. |
August 2008
Attention! MarkPap® Wireless™: Wireless telemedicine for pathology and cytopathology
BioSciCon, Inc. filed a Patent Application with the US Patent and Trademark Office for MarkPap® Wireless. This most recent product of the platform MarkPap® Technology is aimed for wireless transmission of microscopic images from remote parts of the world to a laboratory with professionals for evaluation (telecytopathology). According to most recent estimates, worldwide cell phone usage is expected to reach 3.5 billions connections by the end of this year with a highest annual growth in Asia and Africa. BioSciCon, Inc. continues working tirelessly to fulfill its mission bringing an affordable and accessible cervical cancer screening to all women in the world to save women's lives. To speed up this most recent development, BioSciCon welcomes investments/contributions for co-development of MarkPap® Wireless.
June 2008
Dr. Nenad Markovic and Dr. Olivera Markovic authored the book "What Every Woman Should Know about Cervical Cancer" that has been published by Springer and will be available for purchasing in July 2008. Please visit Springer Web Site.
This book is intended for wide audience: General public, including healthy women at all ages and their spouses, girls and their parents, women suffering from cervical dysplasia, cervical cancer, their families
|
 |
|
and caregivers. It will also well serve students and their instructors at colleges and universities and health professionals involved in women's health.
Recent introduction of HPV vaccines has raised hopes for immunization against cervical cancer and for the first time in the history of humanity for eradication of cervical cancer. The new 'opportunity' has changed many current views on cervical cancer prevention, control, diagnosis and treatment. Many canons and guidelines became subject to review and many revisions are coming. This book is intended to summarize most of these events and to present them in a language understandable for general public. We expect the book will bring all readers the rationale for optimism and will provide guidelines as how to gain knowledge and skills for critical thinking and making educated decision when it will be necessary in their lives.
|
June 2008
BioSciCon Team for Telecytopathology participated at BIO 2008 International Convention. San Diego, California, June 2008 with a poster in the Innovative Corridor. The title of the presentation is A tool for integration of cytology, HPV screening and telecytopathology.
May 2008
An independent study using MarkPap test in cervical cancer screening has been performed in India by the Department of Pathology and the Department of Obstetrics and Gynecology of the All India Institute for Medical Sciences (AIMS), New Delhi, India The paper is entitled Cervical acid phosphatase detection: A guide to abnormal cells in cytology smear screening for cervical cancer. It is published in Indian Journal of Cytology, 25 (1),1-5, 2008.
April 2008
The Global Academy for Women's Health Team participated with an abstract entitled Ask for women' opinion first on FASEB Experimental Biology, 2008. Today's Research: Tomorrow's Health. San Diego, California, April 5-9, 2008.
November 2007
Drs Markovic participated on the 55th Annual Meeting of the American Society of Cytopathology held in Huston on November 2-6, 2007. They presented the results of the application of MarkPap technology and its prospective in telecytopathology.
Please click here to download the abstract. (PDF format)
May 2007
BioSciCon participated with a poster MarkPap Test and HPV vaccination in the Innovation Corridor at the BIO 2007 International Convention held in Boston, MA May 6-9, 2007.
At the Larta Venture Forum 2007, BioSciCon had a business Power Point Presentation "BioSciCon presents MarkPap Technology" given by Mr.Michael Dailey. The Forum that was held on May 1 and 2, 2007 in San Francisco, CA, is a cumulative event of the NIH Commercialization Assistant Program, where the new achievements and prospective of MarkPap technology and BioSciCon national and global business opportunities for investment were presented.
http://www.theventureforum.com/PresentingCompaniesList.asp
April 2007
BioSciCon participated at the Experimental Biology Meeting held in Washington DC, April 18 -May 2, 2007.
Dr. Nenad Markovic presented the concept on cytopathology between art and science, with advantages and problems of color digital imaging and telecytopathology. Dr. Olivera Markovic disclosed the first results of the survey how women feel about getting their samples for Pap test at home, and provided prospective on a Home Pap Test.
March 2007
During the course of the NIH Commercialization Assistance Program 2006/2007, BioSciCon presented its business opportunity at the Larta NIH-CAP Outcome Workshop, March 26-30 in Washington, DC.
Dr. Olivera Markovic, Founder of BioSciCon was honored among entrepreneurs 2007 on the Women in Bio 5th Annual Dinner,
"Innovation Without Borders" that was held in Washington DC on March 27, 2007.
December 2006
Local, national and international citations for BioSciCon:
BioSciCon at FITCI
http://www.platinumpublicrelations.com/pdf/06REL-March-BioSciCon%20at%20FITCI.pdf
BioSciCon listed as a corporate partner in World Science
http://www.world-science.net/x04.htm
October 2006
Dr. Nenad Markovic presented a poster entitled MarkPap® technology, a new business opportunity for the Mid-Atlantic biotechnology in the Innovation Corridor at the Mid-Atlantic 2006 BIO in Washington DC.
September 2006
BioSciCon, as NIH/SBIR phase 2 grant recipient, was selected by the National Institutes of Health, Bethesda, MD to participate in 2006 NIH Commercialization Assistance Program, to foster commercialization of the MarkPap technology. Larta Institute from Los Angeles, CA was assigned to this program.
April 2006
Drs. Markovic participated at the Annual Meeting of the American Association for Cancer Research (AACR). They presented the biomarker-based MarkPap Digital technology, which allows the MarkPap test to be performed in a small laboratory by low-trained technician with easy-to-use MarkPap Kit. Guided by marker-positivity, the technician selects suspicious microscopic fields and transmits the images in diagnostic centers for evaluation by professionals. The results could be returned within hours. The MarkPap Digital opens a prospective for cytological screening around the world.
The same prospective for global cervical cancer control with MarkPap technology was presented at the Innovative Corridor, BIO 2006 Convention in Chicago, IL.
March 2006
Drs. Markovic participated at the Annual Meeting of the American Federation for Clinical Research (AFCR), Clinical Research 2006, held in Washington, DC. They presented their results on the application of the MarkPap test on HPV positive specimens. Since MarkPap could detect HPV infected cells together with cytological signs of abnormality, it was discussed of exploring this test as a potential method for monitoring the success of the HPV vaccination.
At the Clinical Research 2006, Drs. Markovic also announced the prospective of moving Pap test to be performed in doctor's offices, when the new MarkPap Digital will be developed.
Drs. Markovic actively participated on the NIH/NCI organized 4th Early Detection Network Scientific Conference held in Philadelphia. They presented the principle and the pilot results of MarkPap biomarker-based telecytopathology.
December 2005
From June through December 2005, BioSciCon started systematic research
and development of its second generation product MarkPap® Digital
in collaboration with New Hope Pharmaceuticals, Inc., Gaithersburg, MD;
GT Vision, Inc., Hagerstown, MD and ComputerCraft, Inc., McLean, VA. Because
of the presence of the biomarker (the hallmark of MarkPap technology),
which is amenable for digital photography and image analysis, MarkPap
test is qualified for telecytopathology. This is our new product MarkPap®
Digital, a potential integrated device combining image acquisition, image
delivery and image analysis modules. MarkPap® Digital will provide
an opportunity the slides to be stained in one location and the images
from the microscopic field to be transmitted into a center for evaluation.
This practically means that a general laboratory technician (in a remote
laboratory or a nurse in a doctor’s office) can stain a specimen
using the MarkPap® kit and will look on the specimen under the microscope
searching only for “red” cells (marker positive--abnormal
cells). Then, he/she will capture images with a digital camera (mounted
on the microscope) and will transmit them via Internet to the center with
pathologist/cytotechnologist for evaluation. The result may be sent back
electronically within hours.
The development of another product MarkPap Self, which allows a woman
to take a specimen in the privacy of her home, is also in progress. Please
see
http://www.bioscicon.com/markpapproducts.html.
November 2005
BioSciCon’ poster, “MarkPap System. Current status and prospective”
was presented at the Annual Meeting of the American Society for Cytopathology
in San Diego, CA in November, 2005
September 2005
BioSciCon web site is cited at and is linked with the World Science
web site: http://world-science.net/home/links.html
August 2005
Another Success Story about BioSciCon appeared on the SCORE DC Chapter web
site. Please click here to access this story.
July 2005
Read more about BioSciCon presentation on the Century Club Breakfast
at the Business Alliance George Masson Press Release of June 9, 2005
http://www.businessalliance.org/grubstake-presenters.html.
and
http://www.businessalliance.org/grubstake-news.html
.
June 2005
Dr. Olivera Markovic and Dr. Nenad Markovic participated in the Convention
BIO 2005, held in Philadelphia, PA . They presented a poster in
the Innovative Corridor with co-authors Dr. Shelly Parr and Dr. Vojislav
Zizic. The abstract "MarkPap® technology for cervical cancer
control" can be found at:
http://www.bio.org/events/2005/poster/posteronline/index.asp
BioSciCon was selected by the Century Club of George Mason University
for a business presentation at the Century Club Grubstake Breakfast.
The presentation took place on June 9 at Hilton, McLean, VA.
Dr. Nenad Markovic presented the Company and its products. The PP
Presentation can be viewed at the Publication
Page (publication # 17)
May 2005
The PP presentation "MarkPap® test for cervical
cancer screening" by Dr Nenad Markovic, Dr. Olivera Markovic, Dr.
Edward Henderson and the Cervical Cancer Study Group, from ASCO National
Meeting 2005, can be viewed by clicking here-------
www.asco.org/ac/0,1003,_12-002511-00_18-0034-00_19-004969,00.asp
On May 18, 2005, BioSciCon, Inc. Rockville, MD, received the
prestigious SCORE "Small Business Client of the Year 2005
Award." This
award was presented to Dr. Olivera and Dr. Nenad Markovic at the Annual
SCORE Luncheon at the Wyndham Hotel in Washington, DC in the presence
of SCORE Chapter 1 counselors, highest SBA officials and guests. BioSciCon
was awarded in recognition of a highly successful company, and for its
achievements in the development of new products for cervical
cancer screening and prevention that will improve global healthcare and
save women's lives.
March 2005
BioSciCon is announcing the incorporation of a business development
arm:
MarkPap, LLC
The mission of MarkPap, LLC is to commercialize the MarkPap® technology
products. The LLC is accepting applications for new members and for the
management team. If interested, please contact us at info@bioscicon.com.
More information to follow.
February 2005
News from BioSciCon Research and Development.
BioSciCon is currently
developing the MarkPap® Digital and the MarkPap® Self
tests.
MarkPap® Digital is the MarkPap® test combined with IT technology
into a system for telecytopathology, based upon the biomarker. MarkPap® Digital
is intended for low-resource countries, where a general technician
can locally perform the simple MarkPap® test with the easy-to-use kit, make
digital
images of "marked" microscopic fields, and transmit them via
internet for evaluation. This is a chance for women in remote parts of
the world to have an affordable cervical cancer screening within hours.
MarkPap® Self
is also based on the same competitive advantage: The presence of the biomarker
on abnormal cells. Since the biomarker is a chemical compound
deposited inside the cytoplasm, it is not a subject of deterioration like
cellular integrity/morphology. We have seen retained red biomarker
even in parts of cytoplasm after cell disintegration. This is a basis of
prospective
use of MarkPap® test in vaginal specimens of self-obtained material
(MarkPap® Self
Test). This test could be particularly important for women who do not have
access to doctor‚s offices, yet to have cervical cancer screening.
We
believe that MarkPap® technology should revive the cytological approach
to cervical cancer screening and will provide to the rest of the world
the same benefit that Pap test has given to the developed countries.
MarkPap® technology is compatible with other technologies for cervical
cancer screening.
January 2005
We have recently completed three clinical
trials. We compared a combination (marker+Pap) versus Pap alone,
and found that the combination is
superior for detection of true positive/abnormal specimens (up
to 50%), for reduction of false-negatives (more than 50%), and has
equivalent
specificity with the control Pap test. The presence of the biomarker
reduced the screening time from 6 min to 3 min per slide. A total
of 2,000 subject/specimens were analyzed by subgroups including:
low- risk general population, high-risk population, Pap smear control
for MarkPap® smears, and ThinPrep Pap test control for liquid-based
MarkPap® test.
BioSciCon Press Release 2004
December 2004
We are proud to announce the development of two new Research Sites in Los
Angeles, CA, US. BioSciCon is also developing two new International Research
Sites in New Delhi, India and in Belgrade, Serbia and Montenegro. More information
will follow.
October 2004
BioSciCon started exploring equity investment options with local angels
and VCs in order to secure financing for the regulatory study required from
FDA approval for the US market, and the commercialization of the technology.
For more information please contact us at info@bioscicon.com, or call 1-301-610-9130.
August 2004
BioSciCon is finalizing phase II/III clinical laboratory trials
of the MarkPap® Test.
We are currently searching for funding partnership (with individuals or other
enterprises) that will assist us in bringing the new product to market.
July 2004
A paper by Markovic O, Markovic N, and BioSciCon's Cervical Cancer Study
Group: Cervical acid phosphatase: A biomarker of cervical dysplasia and
potential surrogate endpoint for colposcopy appeared in Disease Markers 19(6), 279-286,
2003/2004. The abstract and the full text are available at:
http://www.ncbi.nlm.nih.gov/entrez/query
SBIR
and STTR Success Story for BioSciCon, Inc.
http://grants1.nih.gov/grants/funding/sbir_successes/155.htm
June
2004
Markovic N., Markovic, O. Markovic, J. Sundeen, W. Smith Jr., Markovic
S., BioSciCon Cervical Cancer Research Group*: Pap test and new
biomarker-based technology for enhancing visibility of abnormal cells. 40th American
Society for Clinical Oncology Annual Meeting, ASCO 2004, New Orleans,
Louisiana. June 2004. J Clin Oncol, 22,9561,2004 www.asco.org/ac/1,1003,_12-002636-00_18-0026-00_19-003023,00.asp
Drs.
Nenad and Olivera Markovic participated in the 3rd Annual Early Detection
Research Workshop (EDRN) organized from NCI, NIH in Bethesda,
MD. They presented the development of MarkPap® Technology: From
idea to commercial products. A biomarker-based, in vitro diagnostic
device with potentials for cervical cancer screening. Book of Abstracts
P-28, 2004.
Drs. Olivera and Nenad Markovic participated in the 6th
Annual SBIR/STTR Conference (June 23-24, 2004), organized by NIH in
Bethesda, MD. They
presented, as NIH SBIR 2nd phase awardees, their translational research
entitled MarkPap® Technology for cervical cancer screening. Abstract
Book p.7, 2004.
May 2004
Dr. Lewis Townsend, President Contemporary Women's Health Associates,
Bethesda MD and Drs. Olivera and Nenad Markovic attended the 52nd Annual
Clinical Meeting of the American College of Obstetricians and Gynecologists
in Philadelphia, PA. They presented the results on MarkPap® test
reduces false-negative rates of cervical cancer screening. Townsend L, Markovic
O, Markovic N, BioSciCon Cervical Cancer Study Group*, Obstetrics and
Gynecology, Suppl. 103, (4), 81S,2004.
*BioSciCon Cervical Cancer Study
Group: Parr Shelley, MD., Ross Alan, MD., Sundeen James, MD., Rosser
Rufus, MD., Gould Jed MD., Posarac
Vladan, MD., Mrs. Linda Townsend. April 2004
Dr. James Sundeen, President Diagnostic Pathology Services, Clarksburg,
MD and Medical Director Department of Pathology Prince George Hospital,
Cheverly, MD attended the American Federation for Medical Research
Clinical Research Meeting in Chicago, IL. He presented the results
on Cervical Acid Phosphatase and PreservCyt®. Markovic N, Markovic
O, Sundeen J, Smith W.Jr: Inv Medicine 52, Suppl 2,80,2004.
Drs. Olivera
and Nenad Markovic and Dr. Christopher Kemp, President, Kemp Technologies,
Frederick, MD, attended the Experimental Biology
Meeting 2004 in Washington, DC. They presented the results on: Could
the enzyme-enhanced Pap test be automated? Markovic O, Markovic N,
Kemp C: The FACEB J., Experimental Biology 2004, Abstracts Part 1,
A200.
Another poster, A cytoplasmic biomarker for liquid-based Pap was presented on the same meeting. Markovic O, Markovic N, Sundeen
J.,
Smith W. Jr:
The FACEB J. Experimental Biology 2004, Abstracts Part 1, A200.
March, 2004
Dr. William Smith, Director, Department of Pathology, Suburban Hospital,
Bethesda, MD attended the 2004 Biennial Meeting of the American Society
for Colposcopy and Cervical Pathology held in Disney's Contemporary
Resort, FL, March 2004. He presented the MarkPap® System for cervical
cancer screening. Smith W Jr, Markovic N, Markovic O, Kumar A, Bermeyo,
Mollish P, late Morris M: ASCCP 2004 Biennial Meeting. Book of Abstracts,
p.340.
February, 2004
Dr. Olivera Markovic attended the Symposium on Predictive Oncology & Intervention
Strategies in Nice, France, February 7-10, 2004. She presented the
following scientific communications:
Enhancing Pap test with a new
biological marker of cervical dysplasia
MarkPap® Kit for cervical
cancer screening
MarkPap® Test applied on specimens from solution
Markovic O, Markovic N: Cancer Detection and Prevention, 2004 Symposium
Volume, S-53(21), S-149(318,319). See also
http://www.cancerprev.org/Meetings/2004/Symposia/992/318
January,
2004
Cervical acid phosphatase: A new biomarker of cervical dysplasia appeared
in Arch Oncol 11,243-247,2003. Authors Dr. Olivera Markovic and Dr.
Nenad Markovic, with the members of the BioSciCon Cervical Cancer Study
Group: Dr. James Sundeen, Dr. William Smith, Dr. Shelley Parr, Dr.
Lewis Townsend, Dr. Allan Ross, Dr Rufus Rosser, and Dr. Jed Gould.
| Forward Looking Statement |
DISCLAIMER |
|
Except for historical information provided herein these press releases may contain information and statements of a forward-looking nature concerning the future performance of the Company.
These statements are based on suppositions and uncertainties as well as on management's best possible evaluation of future events. As a result, readers are advised that actual results may differ from expected results.
|
|

